


Next: 4 Conclusion
Up: Using Tritium and x-ray
Previous: 2 High Energy X-ray
As disussed in the introduction, tritium has two advantages over an
x-ray tube: (1) As a radioactive source it is very stable. Since some
typical tritium strengths are a fraction of a Curie, the fractional
fluctuation level should be about 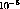 per second. (2) Since
commercial x-ray tubes incorporate windows their emitted beams contain
very few low energy photons. Thus, these sources are inefficient for
fluorescing low Z targets. On the other hand, an unsealed tritium
source emits a rich population of low energy electrons. In essence,
tritium beta particles shining upon a target act as an electron-impact
tube in which the electron spectrum is broad band. These electrons are
not only efficient for creating inner shell ionizations for low Z
atoms, but create them very near the surface so there is very little
self absorption of the emitted k-alpha x-rays. This increased
efficiency becomes important for low Z materials since their
fluorescent yield is increasingly small.
per second. (2) Since
commercial x-ray tubes incorporate windows their emitted beams contain
very few low energy photons. Thus, these sources are inefficient for
fluorescing low Z targets. On the other hand, an unsealed tritium
source emits a rich population of low energy electrons. In essence,
tritium beta particles shining upon a target act as an electron-impact
tube in which the electron spectrum is broad band. These electrons are
not only efficient for creating inner shell ionizations for low Z
atoms, but create them very near the surface so there is very little
self absorption of the emitted k-alpha x-rays. This increased
efficiency becomes important for low Z materials since their
fluorescent yield is increasingly small.
Tritium is a radioisotope of hydrogen whose nucleus contains one
proton and two neutron. It has a half life of 12.32 years, and decays
via 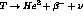 . Tritium is the
lowest energy beta emitter known with a total transition, or endpoint,
energy of 18.6 keV. The beta energy spectrum is easily described by
the Fermi theory[11] as
. Tritium is the
lowest energy beta emitter known with a total transition, or endpoint,
energy of 18.6 keV. The beta energy spectrum is easily described by
the Fermi theory[11] as 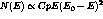 where
E and p are the electron energy and momentum respectively. C is the
Coulomb correction term given by
where
E and p are the electron energy and momentum respectively. C is the
Coulomb correction term given by 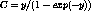 where
where
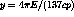 The mean decay energy is 5.685 keV, and a most
probable decay energy of 3.8 keV.
The mean decay energy is 5.685 keV, and a most
probable decay energy of 3.8 keV.
Since our CCD calibration environment prohibits the use of tritium in
a gaseous state (since the CCD requires a cold vacuum environment, and
any membrane or window containing tritium gas would greatly absorb low
energy electrons or x-rays) a solid form is needed. (Cryogenic tritium
is possible but deemed too difficult to handle). This leaves a metal
tritide as the leading candidate since typical source strengths
required for adequate calibration purposes are on the order of 1 Ci.
One Ci corresponds to 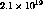 tritium atoms, or about
tritium atoms, or about 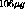 . If the metal to tritium ratio is 1.6 (theoretical maximum),
then for titanium we need about 1.2 mg. For a surface area of about
. If the metal to tritium ratio is 1.6 (theoretical maximum),
then for titanium we need about 1.2 mg. For a surface area of about 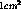 this corresponds to a thickness of about 2.7 microns. The only
large manufacturer remaining in North America capable of making
tritium sources this strong is Safety Light Corp[12], whose
primary application is with the airline industry in tritium loaded
signs for aircraft safety illumination. The geometry of each source is
that of a disk with an outer diameter of 3/4'' and an inner hole of
diameter 1/2''. This gives a surface area of
this corresponds to a thickness of about 2.7 microns. The only
large manufacturer remaining in North America capable of making
tritium sources this strong is Safety Light Corp[12], whose
primary application is with the airline industry in tritium loaded
signs for aircraft safety illumination. The geometry of each source is
that of a disk with an outer diameter of 3/4'' and an inner hole of
diameter 1/2''. This gives a surface area of 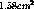 and a
tritium thickness of 1.8 microns. Each sample consists of a titanium
tritide layer on one surface of a 0.010'' thick copper substrate. The
nominal activity densities are
and a
tritium thickness of 1.8 microns. Each sample consists of a titanium
tritide layer on one surface of a 0.010'' thick copper substrate. The
nominal activity densities are 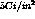 for a total source strength
of 1.25 Ci. The source is manufactured by first evaporating a thin
layer of metal (e.g. titanium) onto a cleaned substrate. The layer's
thickness determines the amount of of final tritium activity. Then the
substrate and metal are heated in a vacuum to a high temperature (e.g.
920
for a total source strength
of 1.25 Ci. The source is manufactured by first evaporating a thin
layer of metal (e.g. titanium) onto a cleaned substrate. The layer's
thickness determines the amount of of final tritium activity. Then the
substrate and metal are heated in a vacuum to a high temperature (e.g.
920  C) and tritium gas is introduced, which absorbs into the
hot metal in about 10 minutes. The temperature is slowly lowered in a
helium atmosphere thereby locking the tritium into the metal. The
essential physics is that hydrogen absorption and permeability are
strongly temperature dependent.
C) and tritium gas is introduced, which absorbs into the
hot metal in about 10 minutes. The temperature is slowly lowered in a
helium atmosphere thereby locking the tritium into the metal. The
essential physics is that hydrogen absorption and permeability are
strongly temperature dependent.
A holder for the tritium samples has been designed and built as shown
in Fig. 18. It is made from 1.25'' diameter iron rod. As shown, beta
particles from tritium illuminate a disk-shaped target, whose emitted
characteristic x-rays can shine through the central aperture and form
a beam. Powerful neodymium magnets surround the long portion of the
aperture, creating a magnetic ``gate'' that stops backscattered (and
the few Compton scattered) electrons from striking the CCD. A magnet
underneath the target helps to guide the incident betas and increase
efficiency. The x-ray output chamber is baffled to help prevent
secondary emission and x-ray scattering. The outside material is made
from soft iron to help guide the magnetic fields. The unit is
designed for replaceable thin targets with a 15 mm diameter. A
calculation shows that the effective or average solid angle subtended
by the central target area from the entire tritium surface is
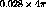 .
.
Targets ranging from beryllium to copper have been tested in the
tritium cup. High Z targets are inefficient for K emission
due to strong competition by L emission. Very low Z targets are also
inefficient because of their fluorescent yield. The CCD calibration
program uses targets of polyethylene, boric oxide, and lithium
fluorine to produce carbon, oxygen, and fluorine x-rays, respectively,
to complement the higher energy x-rays produced by HEXS. Figures 19 -
21 are log plots of the spectra from these three targets. The total
exposure time for the oxygen and fluorine targets is about 40 hours,
spread over 100 days. The carbon data exposure time is about 10 hours.
These spectra are from the same CCD used in the HEXS presentation.
Many features are observed in addition to the main peaks at 277, 525,
and 677 eV. Most prominent is the bremsstrahlung continuum. The
titanium K
emission
due to strong competition by L emission. Very low Z targets are also
inefficient because of their fluorescent yield. The CCD calibration
program uses targets of polyethylene, boric oxide, and lithium
fluorine to produce carbon, oxygen, and fluorine x-rays, respectively,
to complement the higher energy x-rays produced by HEXS. Figures 19 -
21 are log plots of the spectra from these three targets. The total
exposure time for the oxygen and fluorine targets is about 40 hours,
spread over 100 days. The carbon data exposure time is about 10 hours.
These spectra are from the same CCD used in the HEXS presentation.
Many features are observed in addition to the main peaks at 277, 525,
and 677 eV. Most prominent is the bremsstrahlung continuum. The
titanium K and K
and K lines from Compton scattering
off the tritium target are evident at 4511 eV and 4931 eV, as well as
small contaminations at iron and copper K
lines from Compton scattering
off the tritium target are evident at 4511 eV and 4931 eV, as well as
small contaminations at iron and copper K at 6400 and 8048
eV. The continuum reveals the silicon
at 6400 and 8048
eV. The continuum reveals the silicon
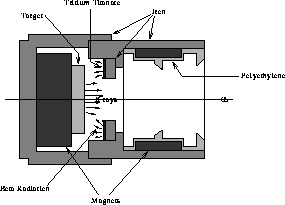
Figure 18: Tritium Source.
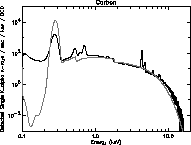
Figure 19: Carbon spectrum from tritium source.
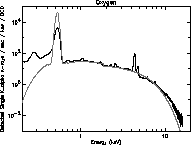
Figure 20: Oxygen spectrum from tritium source.
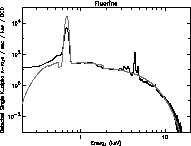
Figure 21: Fluorine spectrum from tritium source.
edge at 1840 eV
and hints at the titanium edge (from the source). Carbon, oxygen and
fluorine contamination is seen with all targets. The family of lines
in the fluorine spectra around 3500 eV are unknown, perhaps a
diffraction effect similar to that discussed with HEXS.
There are two theoretical approaches to understanding the spectra: The
first and easiest uses existing empirical formulas for general x-ray
emission from target materials due to an electron beam of known
energy. This approach is simply an extension of model presented in
section 2, except using an incident electron beam with an energy
spread. Thus we model the beta spectrum from tritium as the sum of
many electron beams with different energies and currents. Such an
assumption can be difficult since the emergent beta spectrum is not
known (although the nuclear emission spectra is well known, the effect
and strength of self-absorption and self-scattering of the beta
electrons in the titanium metal before they emerge as a beam is not
well known). The second approach is more fundamental and uses models
developed by the electron microbeam analysis efforts from the last 30
years.[13] In essence, what are required are (1) the inner shell
ionization cross section for an electron on the target atom, and (2)
the electron slowing down formula.
The theoretical tritium spectra from a model using the first approach
are overlaid in Figs. 19 - 21 as solid lines. Unlike the HEXS model
spectra, the tritium model spectra are normalized to give a good
overlap to the data, using factors of about 2. This is probable due to
uncertainties in the source strength, and self absorption effects.
The fit around the silicon edge is good, although the general shape of
the high energy tail diverges above 10 keV. Also, the absolute value
of K emission is different by factors of three to ten. This
discrepancy may reflect limitations in the given x-ray emission model
at low energy.
emission is different by factors of three to ten. This
discrepancy may reflect limitations in the given x-ray emission model
at low energy.
An indication of the tritium source stability is shown in Fig. 22,
which plots the total detected fluorine K rate observed on
the same CCD over a period of 70 days. The natural decay rate of
tritium predict a 1.1% drop over 70 days, which is far less than the
drop observed at days 60 and 70. The outgassing rate would be even
less. It's possible that a thin layer of material is slowly building
up on the tritium surface, or that the geometry was disturbed
since both the sources and CCDs were removed and reinstalled during
the calibration run. Nevertheless, the short term stability of the
tritium sources is very good.
rate observed on
the same CCD over a period of 70 days. The natural decay rate of
tritium predict a 1.1% drop over 70 days, which is far less than the
drop observed at days 60 and 70. The outgassing rate would be even
less. It's possible that a thin layer of material is slowly building
up on the tritium surface, or that the geometry was disturbed
since both the sources and CCDs were removed and reinstalled during
the calibration run. Nevertheless, the short term stability of the
tritium sources is very good.
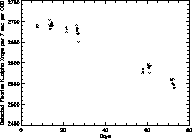
Figure 22: Tritium source stability.
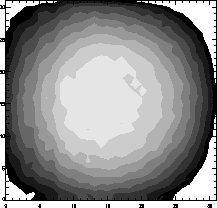
Figure 23: Tritium source uniformity.
The detected fluorine K uniformity is presented in Fig. 23.
Each contour neighboring lever differs by 2%. The circular uniformity
is due to the close proximity of the tritium source to the CCD. With a
10 cm source-CCD distance, the variation of a point source across the
CCD would be about 1%.
uniformity is presented in Fig. 23.
Each contour neighboring lever differs by 2%. The circular uniformity
is due to the close proximity of the tritium source to the CCD. With a
10 cm source-CCD distance, the variation of a point source across the
CCD would be about 1%.
A difficulty in using a tritium source is the slow outgassing of
tritium molecules from the titanium tritide. The migration of
sufficient tritium atoms from the source to the CCD could appear as an
enhanced background, thereby confusing delicate cosmic x-ray
background measurements while in orbit. There are two possible
mechanisms for outgassing. Both start with tritium which migrates from
the bulk through microcracks in the protective oxide layer to sit on
the oxide-vacuum interface. Then (1) tritium atoms can be attracted to
surface defects where they combine to form 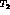 molecules are thereby
leave the oxide surface; or (2) a water molecule from the vacuum
strikes the oxide surface near a tritium atom and an exchange reaction
occurs between a water hydrogen and the tritium atom. To directly
measure the outgassing rate the sources were inserted into a closed
ionization chamber. The slow increase in ionization current was
related to an outgassing rate. Typical measured values at room
temperature were around
molecules are thereby
leave the oxide surface; or (2) a water molecule from the vacuum
strikes the oxide surface near a tritium atom and an exchange reaction
occurs between a water hydrogen and the tritium atom. To directly
measure the outgassing rate the sources were inserted into a closed
ionization chamber. The slow increase in ionization current was
related to an outgassing rate. Typical measured values at room
temperature were around 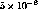 ppd. This value is
consistent with reported values of
ppd. This value is
consistent with reported values of 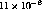 and
and 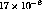 .[14] Attempts were made to reduce the outgassing
rate by evaporating a thin barrier layer onto the source's surface
which was transparent to electrons. On average, a reduction in the
outgassing rate of 3-4 was observed. A reduction of 10 could be
observed by cleaning the surface with methanol and keeping the source
under vacuum. Experimental observations from the CCDs of enhanced
background rates in the vacuum chambers indicated an increase of about
a factor of two, with no subsequent contamination of the CCDs.
Although high, the enhanced background rate was steady over 100 days.
.[14] Attempts were made to reduce the outgassing
rate by evaporating a thin barrier layer onto the source's surface
which was transparent to electrons. On average, a reduction in the
outgassing rate of 3-4 was observed. A reduction of 10 could be
observed by cleaning the surface with methanol and keeping the source
under vacuum. Experimental observations from the CCDs of enhanced
background rates in the vacuum chambers indicated an increase of about
a factor of two, with no subsequent contamination of the CCDs.
Although high, the enhanced background rate was steady over 100 days.
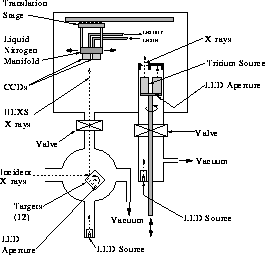
Figure 24: Calibration Chamber.



Next: 4 Conclusion
Up: Using Tritium and x-ray
Previous: 2 High Energy X-ray
sjones@space.mit.edu
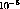 per second. (2) Since
commercial x-ray tubes incorporate windows their emitted beams contain
very few low energy photons. Thus, these sources are inefficient for
fluorescing low Z targets. On the other hand, an unsealed tritium
source emits a rich population of low energy electrons. In essence,
tritium beta particles shining upon a target act as an electron-impact
tube in which the electron spectrum is broad band. These electrons are
not only efficient for creating inner shell ionizations for low Z
atoms, but create them very near the surface so there is very little
self absorption of the emitted k-alpha x-rays. This increased
efficiency becomes important for low Z materials since their
fluorescent yield is increasingly small.
per second. (2) Since
commercial x-ray tubes incorporate windows their emitted beams contain
very few low energy photons. Thus, these sources are inefficient for
fluorescing low Z targets. On the other hand, an unsealed tritium
source emits a rich population of low energy electrons. In essence,
tritium beta particles shining upon a target act as an electron-impact
tube in which the electron spectrum is broad band. These electrons are
not only efficient for creating inner shell ionizations for low Z
atoms, but create them very near the surface so there is very little
self absorption of the emitted k-alpha x-rays. This increased
efficiency becomes important for low Z materials since their
fluorescent yield is increasingly small.
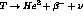 . Tritium is the
lowest energy beta emitter known with a total transition, or endpoint,
energy of 18.6 keV. The beta energy spectrum is easily described by
the Fermi theory[
. Tritium is the
lowest energy beta emitter known with a total transition, or endpoint,
energy of 18.6 keV. The beta energy spectrum is easily described by
the Fermi theory[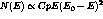 where
E and p are the electron energy and momentum respectively. C is the
Coulomb correction term given by
where
E and p are the electron energy and momentum respectively. C is the
Coulomb correction term given by 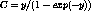 where
where
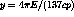 The mean decay energy is 5.685 keV, and a most
probable decay energy of 3.8 keV.
The mean decay energy is 5.685 keV, and a most
probable decay energy of 3.8 keV.
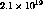 tritium atoms, or about
tritium atoms, or about 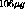 . If the metal to tritium ratio is 1.6 (theoretical maximum),
then for titanium we need about 1.2 mg. For a surface area of about
. If the metal to tritium ratio is 1.6 (theoretical maximum),
then for titanium we need about 1.2 mg. For a surface area of about 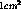 this corresponds to a thickness of about 2.7 microns. The only
large manufacturer remaining in North America capable of making
tritium sources this strong is Safety Light Corp[
this corresponds to a thickness of about 2.7 microns. The only
large manufacturer remaining in North America capable of making
tritium sources this strong is Safety Light Corp[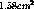 and a
tritium thickness of 1.8 microns. Each sample consists of a titanium
tritide layer on one surface of a 0.010'' thick copper substrate. The
nominal activity densities are
and a
tritium thickness of 1.8 microns. Each sample consists of a titanium
tritide layer on one surface of a 0.010'' thick copper substrate. The
nominal activity densities are 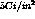 for a total source strength
of 1.25 Ci. The source is manufactured by first evaporating a thin
layer of metal (e.g. titanium) onto a cleaned substrate. The layer's
thickness determines the amount of of final tritium activity. Then the
substrate and metal are heated in a vacuum to a high temperature (e.g.
920
for a total source strength
of 1.25 Ci. The source is manufactured by first evaporating a thin
layer of metal (e.g. titanium) onto a cleaned substrate. The layer's
thickness determines the amount of of final tritium activity. Then the
substrate and metal are heated in a vacuum to a high temperature (e.g.
920  C) and tritium gas is introduced, which absorbs into the
hot metal in about 10 minutes. The temperature is slowly lowered in a
helium atmosphere thereby locking the tritium into the metal. The
essential physics is that hydrogen absorption and permeability are
strongly temperature dependent.
C) and tritium gas is introduced, which absorbs into the
hot metal in about 10 minutes. The temperature is slowly lowered in a
helium atmosphere thereby locking the tritium into the metal. The
essential physics is that hydrogen absorption and permeability are
strongly temperature dependent.
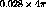 .
.
 emission
due to strong competition by L emission. Very low Z targets are also
inefficient because of their fluorescent yield. The CCD calibration
program uses targets of polyethylene, boric oxide, and lithium
fluorine to produce carbon, oxygen, and fluorine x-rays, respectively,
to complement the higher energy x-rays produced by HEXS. Figures 19 -
21 are log plots of the spectra from these three targets. The total
exposure time for the oxygen and fluorine targets is about 40 hours,
spread over 100 days. The carbon data exposure time is about 10 hours.
These spectra are from the same CCD used in the HEXS presentation.
Many features are observed in addition to the main peaks at 277, 525,
and 677 eV. Most prominent is the bremsstrahlung continuum. The
titanium K
emission
due to strong competition by L emission. Very low Z targets are also
inefficient because of their fluorescent yield. The CCD calibration
program uses targets of polyethylene, boric oxide, and lithium
fluorine to produce carbon, oxygen, and fluorine x-rays, respectively,
to complement the higher energy x-rays produced by HEXS. Figures 19 -
21 are log plots of the spectra from these three targets. The total
exposure time for the oxygen and fluorine targets is about 40 hours,
spread over 100 days. The carbon data exposure time is about 10 hours.
These spectra are from the same CCD used in the HEXS presentation.
Many features are observed in addition to the main peaks at 277, 525,
and 677 eV. Most prominent is the bremsstrahlung continuum. The
titanium K and K
and K lines from Compton scattering
off the tritium target are evident at 4511 eV and 4931 eV, as well as
small contaminations at iron and copper K
lines from Compton scattering
off the tritium target are evident at 4511 eV and 4931 eV, as well as
small contaminations at iron and copper K at 6400 and 8048
eV. The continuum reveals the silicon
at 6400 and 8048
eV. The continuum reveals the silicon




 emission is different by factors of three to ten. This
discrepancy may reflect limitations in the given x-ray emission model
at low energy.
emission is different by factors of three to ten. This
discrepancy may reflect limitations in the given x-ray emission model
at low energy.
 rate observed on
the same CCD over a period of 70 days. The natural decay rate of
tritium predict a 1.1% drop over 70 days, which is far less than the
drop observed at days 60 and 70. The outgassing rate would be even
less. It's possible that a thin layer of material is slowly building
up on the tritium surface, or that the geometry was disturbed
since both the sources and CCDs were removed and reinstalled during
the calibration run. Nevertheless, the short term stability of the
tritium sources is very good.
rate observed on
the same CCD over a period of 70 days. The natural decay rate of
tritium predict a 1.1% drop over 70 days, which is far less than the
drop observed at days 60 and 70. The outgassing rate would be even
less. It's possible that a thin layer of material is slowly building
up on the tritium surface, or that the geometry was disturbed
since both the sources and CCDs were removed and reinstalled during
the calibration run. Nevertheless, the short term stability of the
tritium sources is very good.


 uniformity is presented in Fig. 23.
Each contour neighboring lever differs by 2%. The circular uniformity
is due to the close proximity of the tritium source to the CCD. With a
10 cm source-CCD distance, the variation of a point source across the
CCD would be about 1%.
uniformity is presented in Fig. 23.
Each contour neighboring lever differs by 2%. The circular uniformity
is due to the close proximity of the tritium source to the CCD. With a
10 cm source-CCD distance, the variation of a point source across the
CCD would be about 1%.
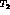 molecules are thereby
leave the oxide surface; or (2) a water molecule from the vacuum
strikes the oxide surface near a tritium atom and an exchange reaction
occurs between a water hydrogen and the tritium atom. To directly
measure the outgassing rate the sources were inserted into a closed
ionization chamber. The slow increase in ionization current was
related to an outgassing rate. Typical measured values at room
temperature were around
molecules are thereby
leave the oxide surface; or (2) a water molecule from the vacuum
strikes the oxide surface near a tritium atom and an exchange reaction
occurs between a water hydrogen and the tritium atom. To directly
measure the outgassing rate the sources were inserted into a closed
ionization chamber. The slow increase in ionization current was
related to an outgassing rate. Typical measured values at room
temperature were around 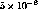 ppd. This value is
consistent with reported values of
ppd. This value is
consistent with reported values of 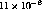 and
and 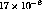 .[
.[